画像 favipiravir malaysia 271134
Favipiravir, an antiviral drug, is being used for COVID19 treatment, and we currently have limited information regarding its efficacy and safety Thus, the present study was undertaken to evaluate the adverse drug events (ADEs) reported in the WHO pharmacovigilance database By Paphamon Arayasukawat BANGKOK (NNT) Thai government is working on buying the "Favipiravir" drug patent in order to stabilize domestic demands During a meeting of the cabinet, Minister of Public Health and Deputy Prime Minister, Anutin Charnvirakul informed the members that Thai Government P Favipiravir (T705) is a purine analogue antiviral approved for use in Japan against emerging influenza strains;
Combination Of Interferon Beta 1b Lopinavir Ritonavir And Ribavirin Versus Favipiravir In Hospitalized Patients With Non Critical Covid 19 A Cohort Study
Favipiravir malaysia
Favipiravir malaysia- KUALA LUMPUR (March 6) 7Eleven Malaysia Holdings Bhd's offer to acquire shares of Caring Pharmacy Group Bhd that it does not own commences today, and will be closed on March 27Both 7Eleven and Caring filed the offer document to the bourse today, for the unconditional mandatory takeover offer by Convenience Shopping (Sabah) Sdn Bhd (CSSSB), a Avigan (Favipiravir) an influenza antiviral developed by FujiFilm, comes in tablet and IV form and is approved in a number of countries as a therapeutic to reduce the severity of COVID19 symptoms and speed the recovery of patients diagnosed with the virus




Coronavirus Dr Reddy S Lab Terminates Trial Study Of Avigan In Kuwait India News
COVID19 infections Favipiravir is launched in China, Saudi Arabia and UAE for the treatment of COVID19 infections As of June 21, Avigan ® is being made available in countries such as Indonesia, Malaysia and Thailand, based on regulatory approval of the therapy for the treatment of COVID19 infections In August , Dr Reddy's Laboratories launched AVIGAN ® (Favipiravir Infection cases total about 187,000 and deaths reached 2,755 as of Wednesday, according to public broadcaster NHK Japan has already approved Avigan, known generically as favipiravir, as an Avigan (Favipiravir) Antiviral Description Avigan (Favipiravir) (T705) (Reeqonus) Tablet is a pyrazinecarboxamide derivative with activity against RNA viruses Favipiravir is converted to the ribofuranosyltriphosphate derivative by host enzymes and selectively inhibits the influenza viral RNAdependent RNA polymerase
Asia Pacific (China, Japan, South Korea, India, Australia, Taiwan, Indonesia, Thailand, Malaysia, Philippines, Vietnam) Latin America (Mexico, Brazil, Argentina) Middle East and Africa (Turkey, Saudi Arabia, UAE) And Others The global Favipiravir market is expected to gain at a desirable rate during the predicated timespan between 21 to 27Favipiravir adalah obat antivirus yang digunakan untuk mengatasi beberapa jenis virus influenza, seperti influenza A, yang menyebabkan flu burung dan flu babi, inluenza B, dan influenza C Saat ini, favipiravir juga sedang diteliti lebih lanjut untuk menangani infeksi virus Corona atau COVID19 Favipiravir atau t705 atau 6fluoro3hydroxy2pyrazinecarboxamide merupakan obat turunan Currently, the Malaysian Consensus Management Guidelines for COVID19 maintains Favipiravir, as the only antiviral therapy for the management of COVID19 cases Even this recommendation may change as more evidence becomes available
Most frequent ADEs suspected to be caused by the favipiravir included increased hepatic enzymes, nausea and vomiting, tachycardia, and diarrhea Severe and fatal ADEs occurred more frequently in men and those over the age of 64 yearsGlenmark Pharmaceuticals appears to have stolen a march over its peers after it received regulatory clearance in India for and launched its version of favipiravir, the antiviral currently being evaluated as part of treatment options for COVID19 in several countries including Japan Glenmark's product, to be sold as FabiFlu, has received emergency use authorization in India for the Favipiravir (T705) is a synthetic prodrug, first discovered while assessing the antiviral activity of chemical agents active against the influenza virus in the chemical library of Toyoma chemicals A lead compound, A/PR/8/34, later designated as T1105, and its derivatives were found to have antiviral activities




A Mini Review On Emerging Targets And Approaches For The Synthesis Of Anti Viral Compounds In Perspective To Covid 19 Bentham Science



Favipiravir Wikipedia
The Southeastern nation of Malaysia recently announced the planning for a major clinical trial to evaluate two low cost, widely available, orally administered generic drugs targeting early onset COVID19, including Favipiravir and Ivermectin"As for Favipiravir, the Health Ministry is getting the drugs from suppliers," said Dr Noor Hisham in a statement on his Facebook page on Saturday In February, Reuters reported that Favipiravir, which is an antiflu drug, is effective in helping coronavirus patients inTo estimate and forecast the market size of Asia Pacific favipiravir market from 19 to 25 and growth rate until 25 To classify and forecast Asia Pacific favipiravir market based on route of administration, dosage form, distribution channel, application, company and regional distribution To identify dominant region or segment in the Asia




Lupin Launches Covid 19 Drug Favipiravir In India At Rs 49 Per Tablet Business Standard News



Combination Of Interferon Beta 1b Lopinavir Ritonavir And Ribavirin Versus Favipiravir In Hospitalized Patients With Non Critical Covid 19 A Cohort Study
MOH Testing Out Two Drugs To Treat Covid19, Says Dr Noor Hisham They said it looks promising, but more testing is needed Subscribe to our Telegram channel for the latest stories and updates The Health Ministry is looking to test two drugs – Ivermectin (IVM) and Favipiravir to treat Covid19 to see their effectiveness against the diseaseFavipiravir side effects Some common side effects of the Fabiflu are swelling all over the body, muscle pain, asthma attack, gastrointestinal upset, miscarriages, hay fever, and allergic dermatitis The use of favipiravir in pregnancy is restricted as it Malaysia Singapore Philippines If approved, the drug, also known as favipiravir, would be the third treatment drug for COVID19, the respiratory disease caused by the virus




Glenmark S Antiviral Drug Favipiravir Boosts Recovery Time Of Covid 19 Patients In Phase 3 Study Covid 19 Hospimedica Com




Avigan Trial To Stretch Beyond June As Japan S Coronavirus Cases Plunge Nikkei Asia
The drug, favipiravir, is sold under the brand name Avigan, and was developed by Fujifilm Toyama Chemical in 14, according to Nikkei Asian Review Around 0 patients in the Chinese cities of (Nikkei) Three days after Beijing endorsed the Japandeveloped flu drug Avigan as a treatment for COVID19, Indonesia is importing millions of doses to treat its patients, President Joko "Jokowi" Widodo said during a livestreamed press briefing from the Presidential Palace on Friday "No antivOn , the Department of Intellectual Property (DIP) of Thailand rejected a Thai patent application for a granulated powder tablet containing 6fluoro3hydroxy2pyrazinecarboxamide, also known as Favipiravir, which is a drug used to treat viral infections including COVID19 Favipiravir has been used in many countries




Malaysian Indian Academic Medical Centers Finds Favipiravir Relatively Safe Somewhat Effective For Covid 19 Patients But More Research Necessary




Indonesia Registers Avifavir Favipiravir For Covid 19 Treatment Coronavirus Today
63 Global Favipiravir Market Sales, Value and Growth Rate by Region 1621 64 Global Favipiravir Sales Forecast by Region 2126 65 Global Favipiravir Market Value Forecast by Region Brief Summary The study aims to investigate the efficacy of favipiravir in highrisk COVID19 patients The study population includes symptomatic mildtomoderate COVID19 inpatients, within first 7 days of illness, who are 50 years old and above, and have 1 or more comorbidities The study is designed as a randomised, openlabel, multicenter Favipiravir, an antiviral drug, has shown promising results in Covid19 clinical studies in countries such as India, China, Japan and Russia and some of



Dr Reddy S Launches Avigan Tablets Priced At 99 Each The Hindu



Malaysia Starts Ivermectin Trial For High Risk Covid 19 Patients Outbreak News Today
A multicentered randomized clinical study led by the Zhongnan Hospital of Wuhan University also suggested that the therapeutic effect of Favipiravir is much better than that of the control group Favipiravir has been recommended to medical treatment teams and should be included in the diagnosis and treatment plan for COVID19 as soon as possible, Zhang saidMalaysia Teoh Cherh Yun, clinical pharmacist, Hospital Sultanah Maliha, Langkawi Favipiravir, developed by a Japanese manufacturer, has already been tested in more than 1 patients with confirmed improvement The Japanese government plans to possibly provide it free of charge to up to 50 countries worldwide, with countries already Efek samping favipiravir diantaranya berupa efek samping hepatik dengan peningkatan enzim, gastrointestinal seperti diare, mual, muntah, dan nyeri abdomen, gangguan hematologi, respiratorik, metabolik, hipersensitivitas, serta efek samping lain seperti vertigo, ekimosis, pandangan kabur, dan polip tonsil




Fujifilm S Avigan Hopes Hit After Japan Puts Covid Drug On Hold Nikkei Asia




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr
Consumer Price Guide (CPG) is a list of medicine prices It serves as a public reference to purchase medicines in the private sector The information provide medicine availability and market price guidance for consumers to make informed choices "The NPRA will provide an expedited review route to bring in the clinical study products to Malaysia as soon as possible, while for Favipiravir, the ministry is sourcing the drug from suppliers,"Favipiravir is a novel antiviral drug approved for influenza treatment in Japan Little is known about favipiravir pharmacokinetics in critically ill patients Here, we report a patient with influenza treated with favipiravir and undergoing continuous venovenous haemofiltration (CVVH) on the Intensi



Apps Who Int Iris Bitstream Handle Who 19 Ncov Remdesivir 1 Eng Pdf




Four Asean Nations Among 38 To Get Avigan Drug From Japan For Clinical Trial The Star
Favipiravir is used for the treatment of advanced Ebola virus infection in a small animal model Favipiravir suppressed the replication of Zaire Ebola virus and prevented a lethal outcome in 100% of the animals Based on the studies, Favipiravir can be a candidate for the treatment of Ebola hemorrhagic fever BTM Favipiravir is quickly emerging as the top choice among 25 drug candidates that the Council of Scientific and Industrial Research (CSIR) is considering for COVID19 treatment, the Ministry of Science and Technology said in a press release yesterday (April 30) Favipiravir is an antiviral drug that was originally made to treat the flu in Japan Favipiravir, an influenza drug available on overseas markets, has been put in a clinical trial in Shenzhen, south China's Guangdong Province, with 70 patients enlisted, he said The initial outcome of the trial shows the drug has relatively obvious efficacy and low adverse reactions



2



Malaysia Reports 80 Percent Of Covid 19 Deaths Had Chronic Diseases Outbreak News Today
The Malaysian and Indian researchers discovered that the most frequently reported ADEs associated with favipiravir involved increased hepatic enzymes, nausea and vomiting, tachycardia and diarrhea There were some occurrences of fatal ADES which although rare, did occur more in men over the age of 64 PUTRAJAYA, March 25 — The Health Ministry is considering the use of antiviral drug 'Favipiravir' to treat Covid19 positive patients, said its directorgeneral Datuk Dr Noor Hisham Abdullah He said the drug, which is also known as 'Avigan' and 'Fuhaikang', was not yet registered in the country "We (MOH) can approve itAnd several phase 2 and 3 clinical trials are




Minimum Costs To Manufacture New Treatments For Covid 19 Sciencedirect




Covid 19 Pharmacotherapy In Asia Latest News For Doctors Nurses And Pharmacists Respirology
Request Download Sample Need of Customization Pricing & Purchase Options New Jersey, United States, The Favipiravir Market research report offers the key analysis of the Favipiravir market situation including best facts and data, definitions, SWOT analysis, expert opinions, and the latest global developmentsThe report also calculated the market size, revenue, BANGKOK (The Nation/ANN) The Food and Drug Administration (FDA) has approved for use in Thailand Favipiravir tablets administered to Covid19 patients researched, developed and manufacturedFDA approved, Avigan (Favipiravir) Tablets, is an antiviral used to manage influenza We have exported in Vietnam, Philippines, Burma, Cambodia, Hong Kong, Indonesia, Japan, Laos, Malaysia, Papua New Guinea, South Korea, Singapore, Thailand, Taiwan Contact us at urgent@cancermedicinesnetworkcom or give us a call / WhatsApp at 91



Grant Of Avigan Tablets To Malaysia Embassy Of Japan In Malaysia
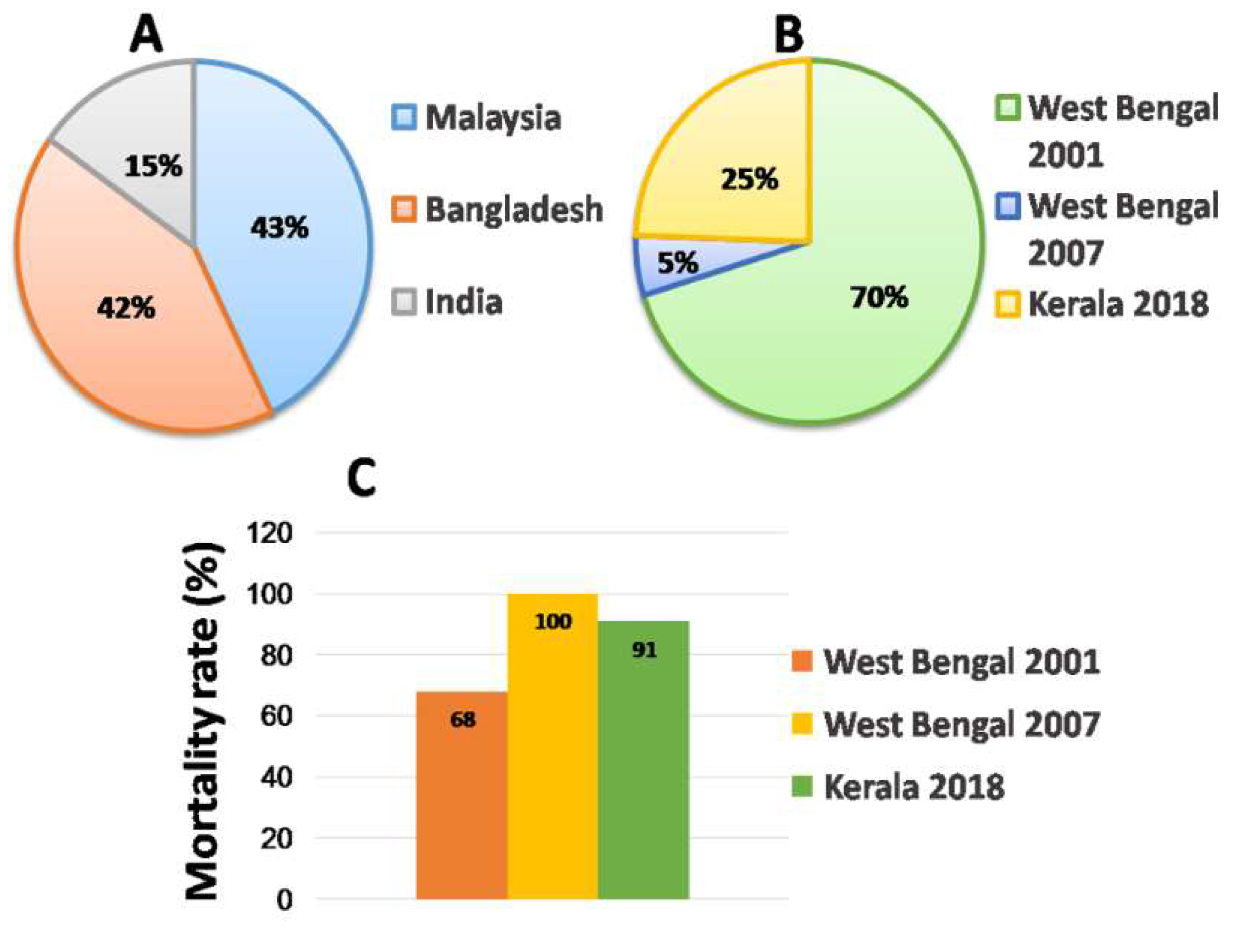



Viruses Free Full Text Nipah Virus Past Outbreaks And Future Containment Html
Favipiravir is a member of pyrazines and a primary carboxamide Discovered by Toyama Chemical Co, Ltd in Japan, favipiravir is a modified pyrazine analog that was initially approved for therapeutic use in resistant cases of influenza The antiviral targets RNAdependent RNA polymerase (RdRp) enzymes, which are necessary for the transcription JAKARTA (THE JAKARTA POST/ASIA NEWS NETWORK) President Joko Widodo said on Monday (March 23) that Indonesia secured a supply of chloroquine, an antimalaria drug being tested as a possible form Myanmar develops its own Covid19 medicine A Myanmar pharma company has produced the first domesticallymanufactured Covid19 medicine, but so far it is only for clinical use for the treatment of early signs of the coronavirus infection and to support a recovery in the patient The drug, named Favipac, is being manufactured by Yangonbased



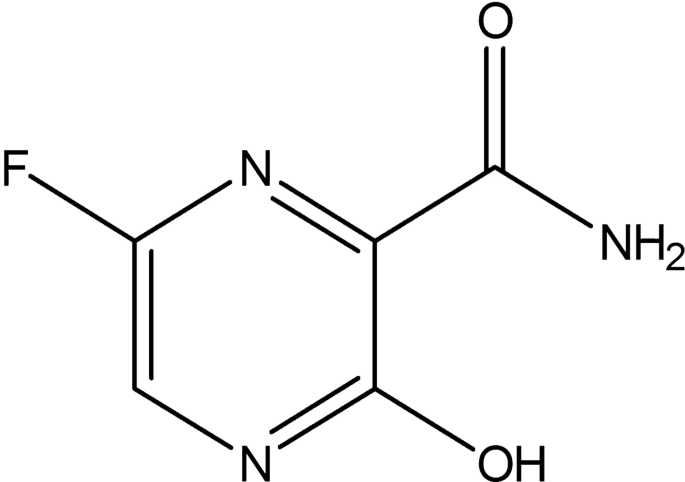
Favipiravir T 705 Rna Polymerase Inhibitor Medchemexpress




Three Types Of Medicine Available In Malaysia Can Be Used To Treat Covid 19 Says Health D G The Edge Markets
On 5/6/21 at 346 AM, snoop1130 said A patent application for Japanesemade antiviral drug Favipiravir has been rejected by Thailand's Intellectual Property Department over its manufacturing quality This is going to take someone smarter than me on Favipiravir was originally developed to treat influenza by Toyama Chemical, which is owned by Fujifilm, the Japanese photography company that now has sizable holdings in biomedicineThe drug wasFavipiravir 0mg Tablet Fabiflu is an antiviral medication It has as of late been endorsed for the treatment of gentle Covid infection (COVID19) It represses a protein considered RNA polymerase that aides the infection makes more duplicates of itself This way it




Glenmark Gets Regulatory Approval For Favipiravir To Treat Covid 19




T 705 Favilavir Favipiravir Cas Number 96 9 Cayman Chemical
Dr Noor Hisham said the government was also committed to ensuring adults in Malaysia achieved herd immunity in line with the target of the National Covid19 Immunisation Programme — Bernama Related Articles Dewan Rakyat Speaker calls on party leaders to rotate attendance of MPs on Covid19 concern;
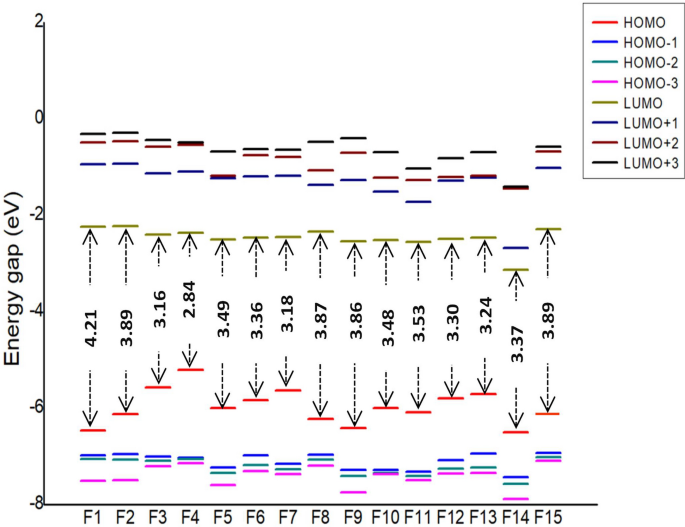



Piperazine Substituted Derivatives Of Favipiravir For Nipah Virus Inhibition What Do In Silico Studies Unravel Springerlink




Avigan Still Awaits Japanese Approval As Coronavirus Cure Nikkei Asia
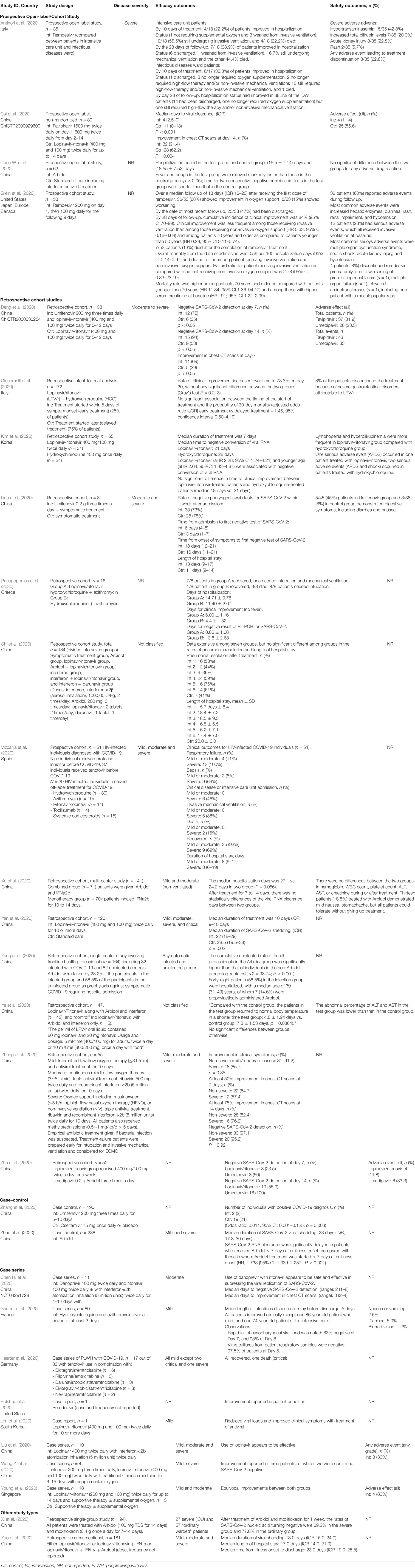



Frontiers Directly Acting Antivirals For Covid 19 Where Do We Stand Microbiology




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr




Japan Review Of Avigan Says Efficacy For Covid 19 Treatment Inconclusive Kyodo




Covid 19 Health Ministry Mulls Use Of Favipiravir Drug To Treat Patients Malaysia Malay Mail




Favipiravir Wikipedia




Biosimilars Regulation Clinical Trials Approval And Adverse Events In Malaysia



Covid 19 Treatment In Moh Facilities Mps Young Pharmacist Chapter
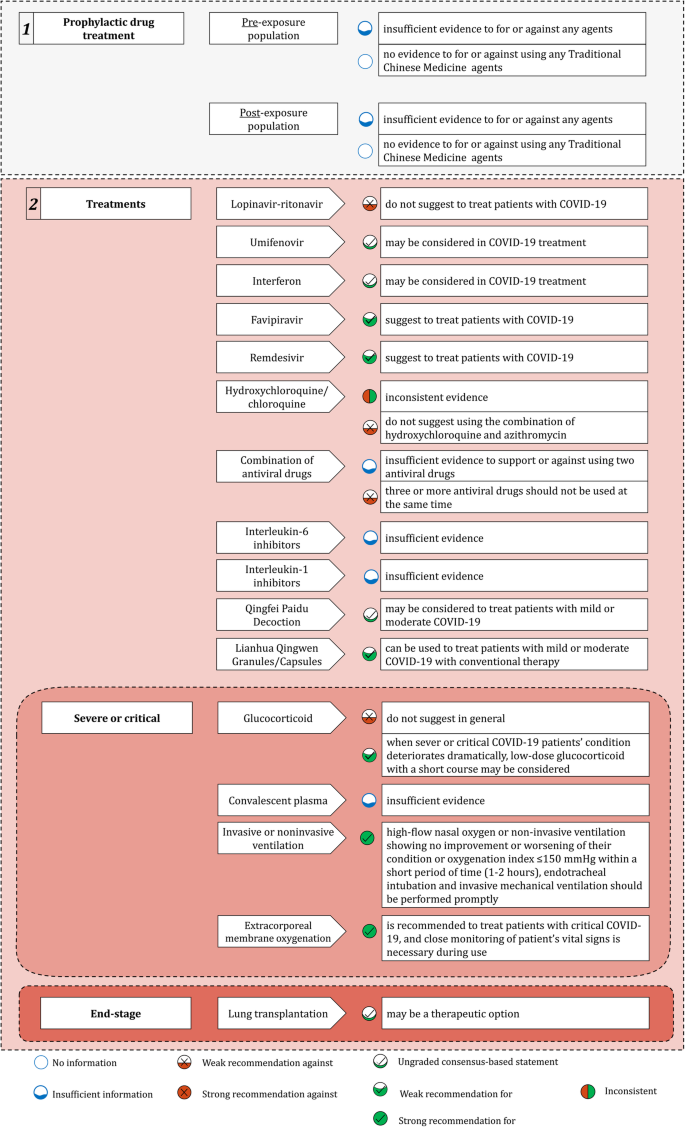



Chemoprophylaxis Diagnosis Treatments And Discharge Management Of Covid 19 An Evidence Based Clinical Practice Guideline Updated Version Military Medical Research Full Text
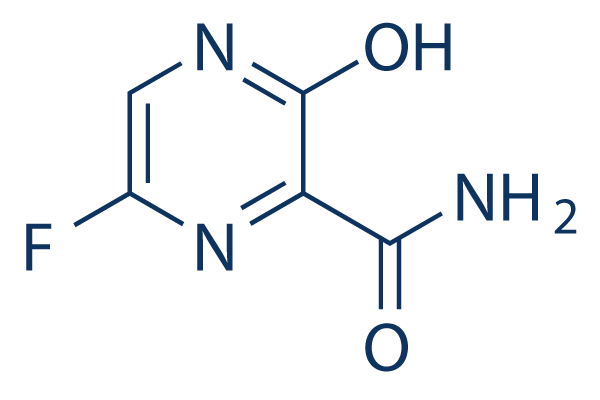



Favipiravir T 705 99 Hplc Selleck Dna Rna Synthesis Inhibitor




Japan Eyes Tripling Avigan Drug Stockpile To Fight Coronavirus




Detecting Falsified Remdesivir
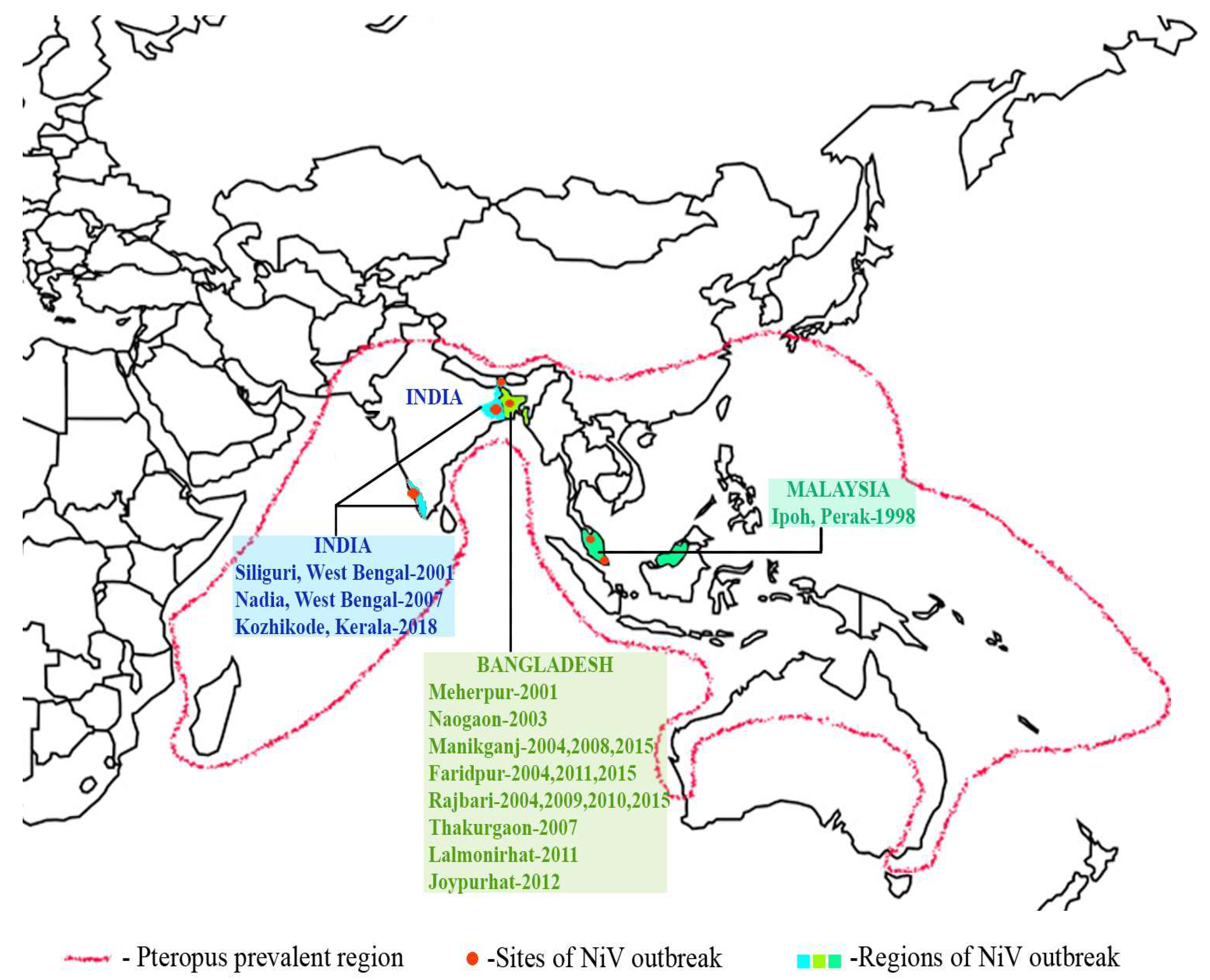



Viruses Free Full Text Nipah Virus Past Outbreaks And Future Containment Html




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr




Avigan Approved For Covid 19 Treatment In Malaysia Agility Logistics




Clinical Characteristics And Risk Factors For Severe Covid 19 Infections In Malaysia A Nationwide Observational Study The Lancet Regional Health Western Pacific




Group Of Malaysian Doctors Officials Suggest A Plan B To Vaccination Centering On Ivermectin




Coronavirus Dr Reddy S Lab Terminates Trial Study Of Avigan In Kuwait India News




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr



Www Thelancet Com Pdfs Journals Lanwpc Piis2666 6065 9 Pdf




Antivirals For Covid 19 A Critical Review Sciencedirect




Antivirals For Covid 19 A Critical Review Clinical Epidemiology And Global Health




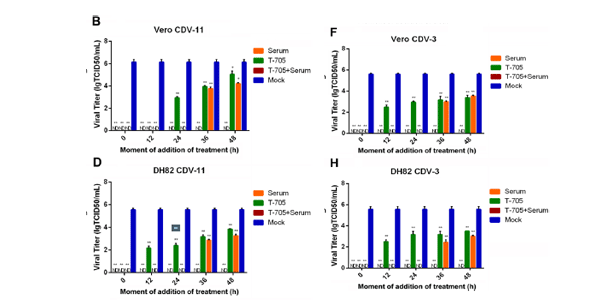
Pdf Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model



Http Medrxiv Org Cgi Reprint 04 10 v3



Malaysian National Health Ministry Plans Clinical Trial To Evaluate Ivermectin Favipiravir As A Low Cost Orally Administrated Treatment For Covid 19




Flu Drug Favipiravir Is Effective In Treating Covid 19 The Edge Markets




Covid 19 Outbreak In Malaysia
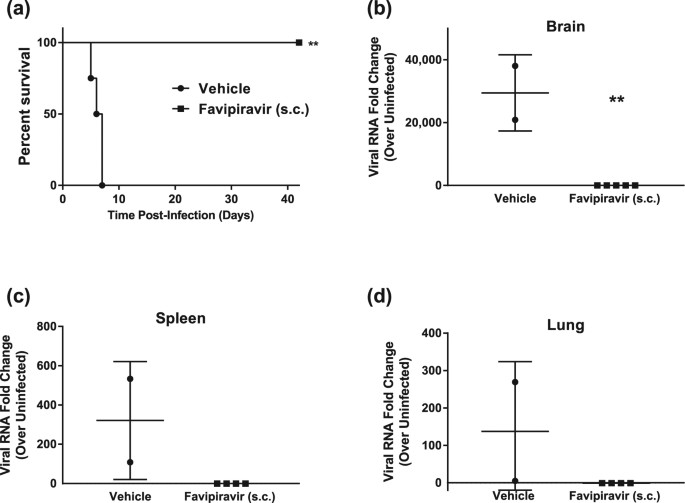



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports
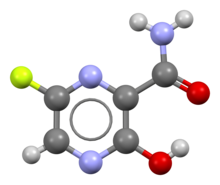



Favipiravir Wikipedia




Avigan Set For Approval This Month In Japan As Coronavirus Drug Nikkei Asia



Frontiers Directly Acting Antivirals For Covid 19 Where Do We Stand Microbiology




19hfaoqbxjhtum




India S Glenmark To Study Potential Covid 19 Drug Combination The Star




Japan To Offer Anti Flu Avigan To 38 Countries As Early As This Week




Fujifilm Accelerates Favipiravir Production For Covid 19 Treatment



Core Ac Uk Download Pdf Pdf




Zhejiang Hisun Shows Positive Effect Of Favipiravir On Covid 19




China Says Japan Developed Drug Avigan Works Against Coronavirus Nikkei Asia




Thai Made Favipiravir Tablets For Covid Treatment Get Fda Approval The Star



Asean Org Storage 02 Covid 19 Report Of Asean Biodiaspora Regional Virtual Center 7october Pdf
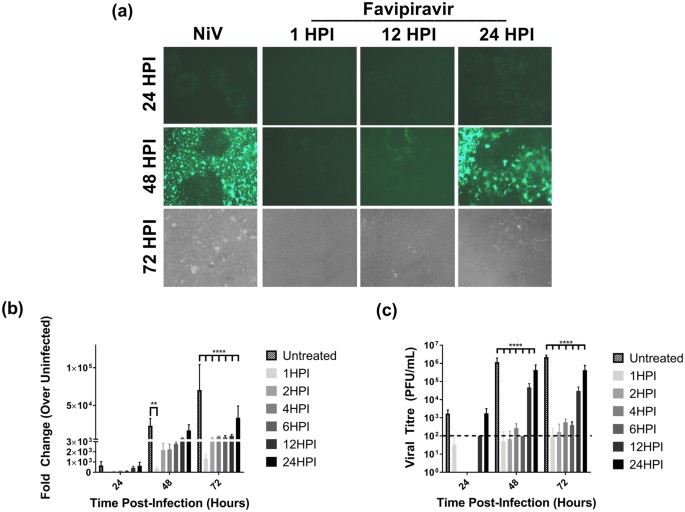



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports
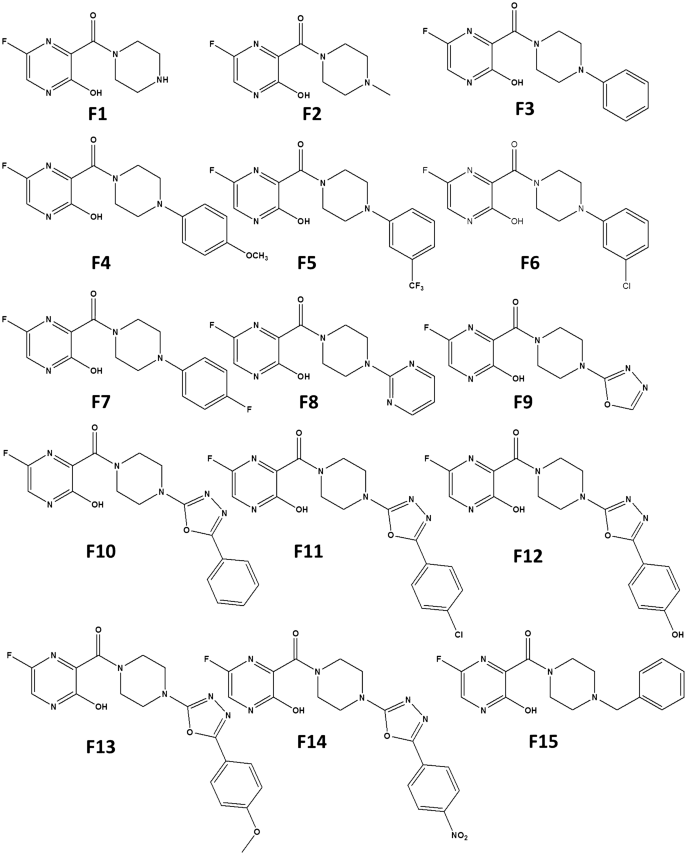



Piperazine Substituted Derivatives Of Favipiravir For Nipah Virus Inhibition What Do In Silico Studies Unravel Springerlink




Minimum Costs To Manufacture New Treatments For Covid 19 Sciencedirect




Pdf Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model




Bernama Covid 19 Health Ministry Mulls Over Use Of Favipiravir To Treat Patients



Piperazine Substituted Derivatives Of Favipiravir For Nipah Virus Inhibition What Do In Silico Studies Unravel Springerlink
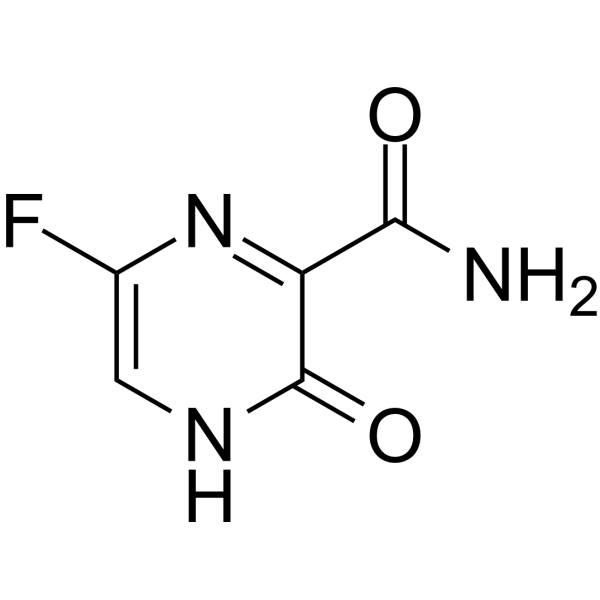



Favipiravir T 705 Rna Polymerase Inhibitor Medchemexpress




Covid 19 Pneumonia Outcomes Similar With Favipiravir Inhaled Interferon Beta Hcq Latest News For Doctors Nurses And Pharmacists Infectious Diseases
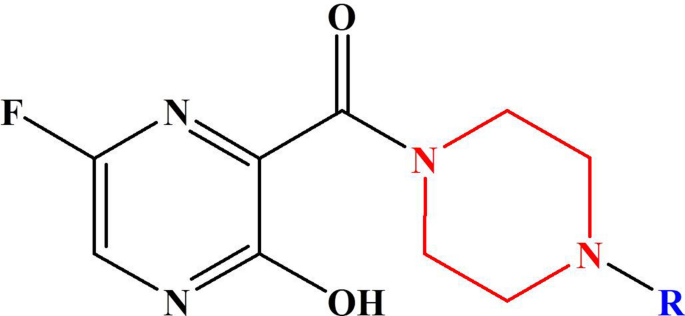



Piperazine Substituted Derivatives Of Favipiravir For Nipah Virus Inhibition What Do In Silico Studies Unravel Springerlink
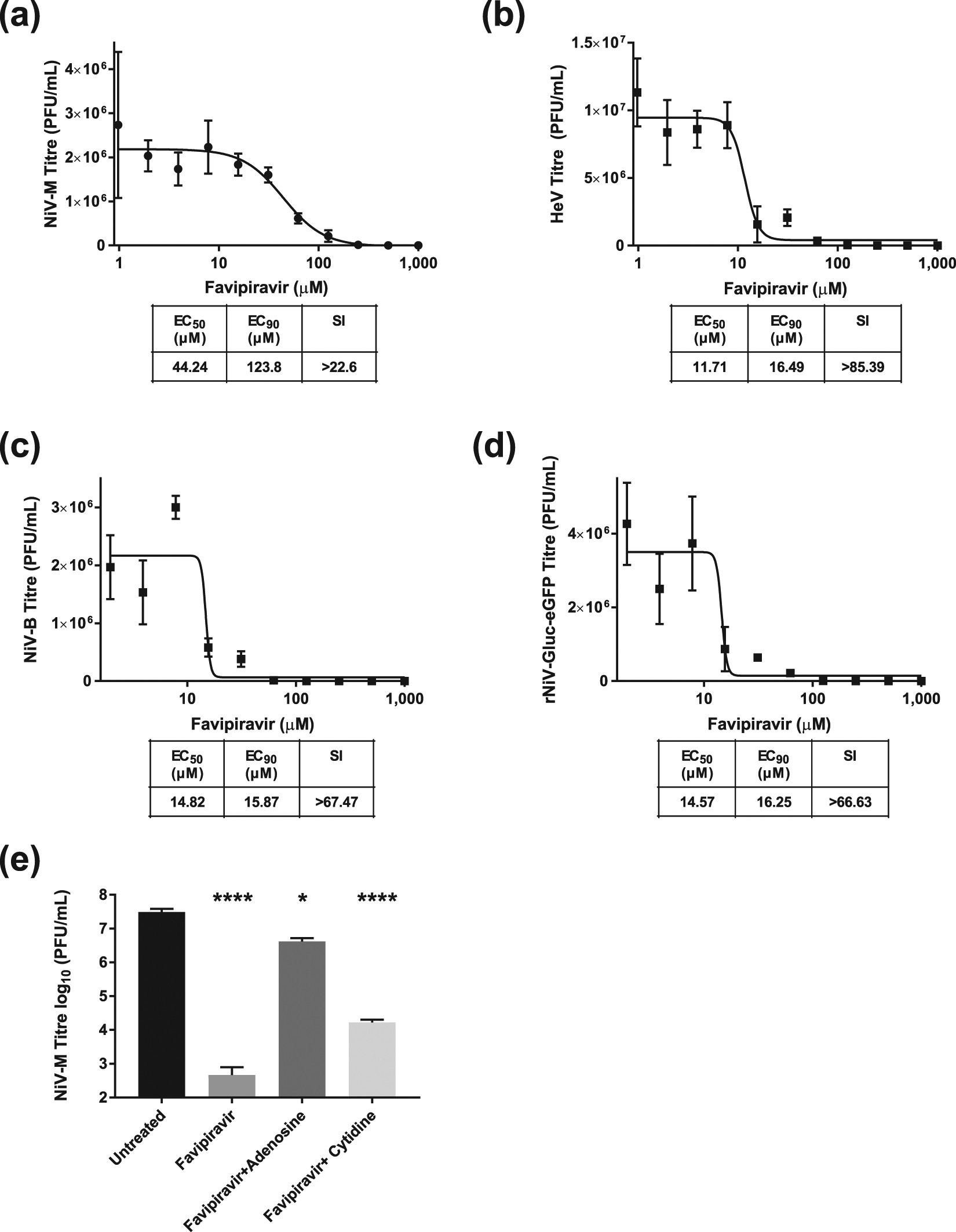



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports




Favipiravir Wikipedia




Malaysian National Health Ministry Plans Clinical Trial To Evaluate Ivermectin Favipiravir As A Low Cost Orally Administrated Treatment For Covid 19



Covid 19 Treatment In Moh Facilities Mps Young Pharmacist Chapter




Covid 19 Treatment Fabiflu By Glenmark Hcq Remdesivir And Other Drugs That Are Being Used To Treat Coronavirus Patients Health News Firstpost




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr
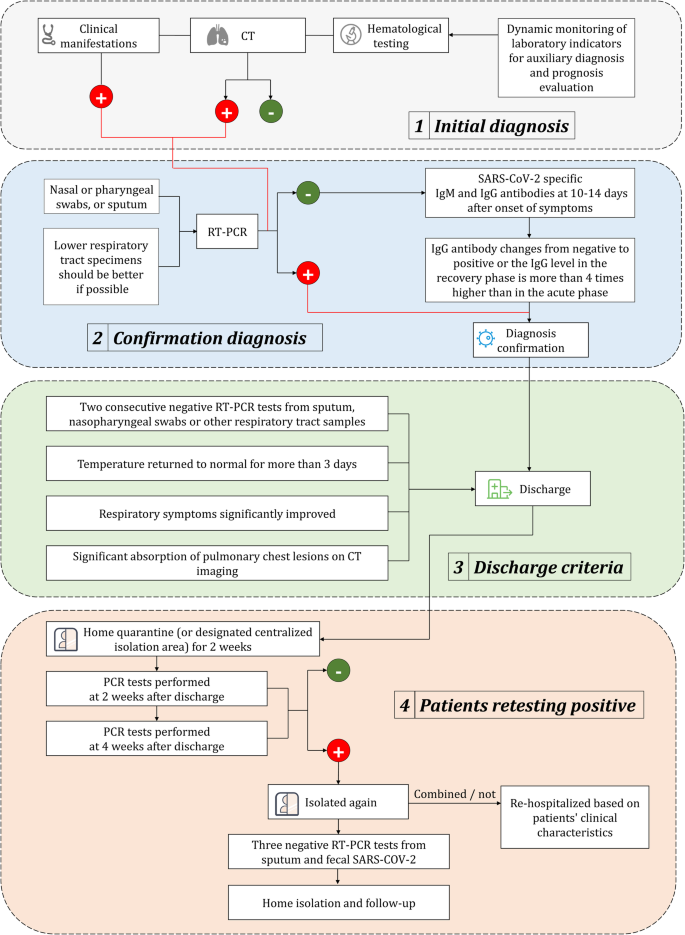



Chemoprophylaxis Diagnosis Treatments And Discharge Management Of Covid 19 An Evidence Based Clinical Practice Guideline Updated Version Military Medical Research Full Text



Covid 19 Moh Gov My Garis Panduan Garis Panduan Kkm Annex 2e Clinical Management Of Confirmed Covid 19 Case In Adult And Peadiatrics Pdf




Covid 19 Health Ministry Mulls Use Of Favipiravir Drug To Treat Patients Malaysia Malay Mail
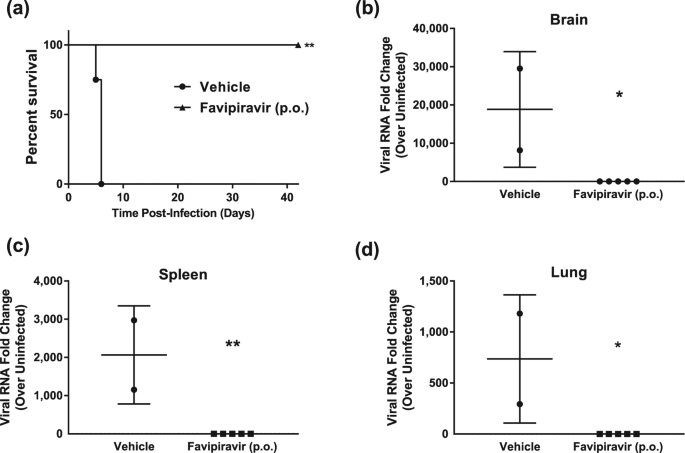



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports



Japan S Fujifilm Starts Avigan Trial To Treat Coronavirus




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr



Avigan Approved For Covid 19 Treatment In Malaysia Maritime Fairtrade




Gastrointestinal Manifestation As Clinical Predictor Of Severe Covid 19 A Retrospective Experience And Literature Review Of Covid 19 In Association Of Southeast Asian Nations Asean Aumpan Jgh Open Wiley Online Library




Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Idr




Pdf Original Favipiravir Use In Covid 19 Analysis Of Suspected Adverse Drug Events Reported In The Who Database




Indonesia Adopts Remdesivir As Malaysia Shops For Covid Vaccines Nikkei Asia



Http Medrxiv Org Cgi Reprint 04 10 v3




Factsheet Written By Dr Phan Chia Wei Head Of Cic On Clinical Trials On Covid 19 Worldwide The Factsheet Is Published By The Academy Of Science Malaysia Asm In Collaboration With The Young




Health D G Malaria Hiv Drugs Can Be Used To Treat Covid 19




Covid 19 Pharmacotherapy In Asia Latest News For Doctors Nurses And Pharmacists Respirology




Favipiravir Wikipedia
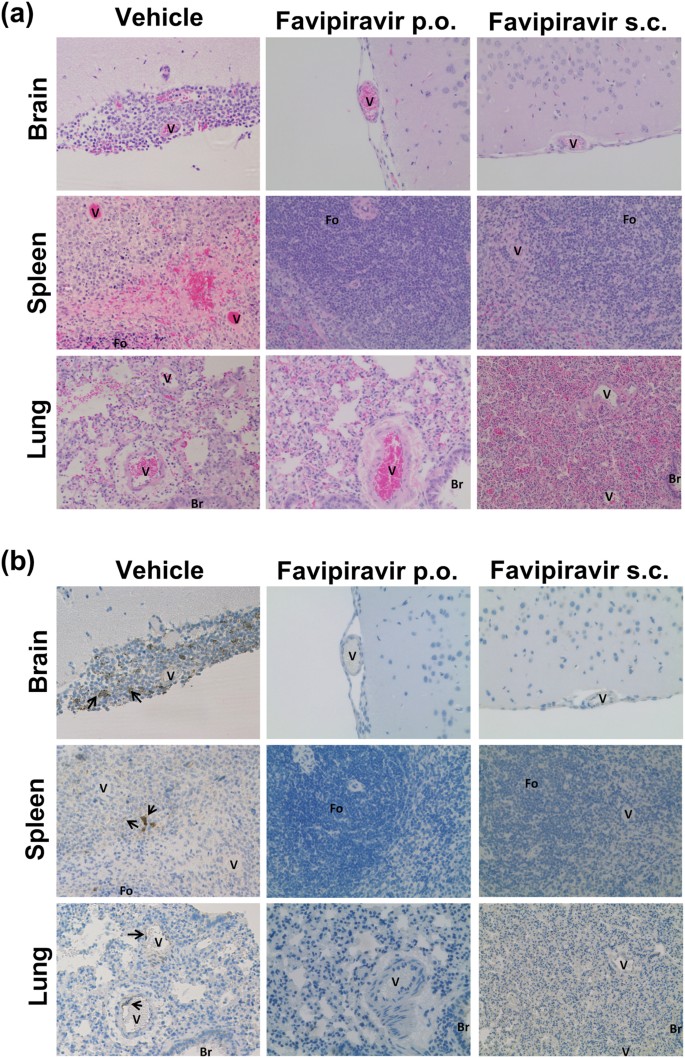



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports




Covid 19 Pharmacotherapy In Asia Latest News For Doctors Nurses And Pharmacists Respirology




Favipiravir Wikipedia




Avigan Shows Promising Results In Treating Covid 19 Patients In Japan


コメント
コメントを投稿